部件
-
2025.09.01
Medtec China|球囊扩张导管的介绍2
咱们废话不多说,继续来更新球囊扩张导管的介绍吧。
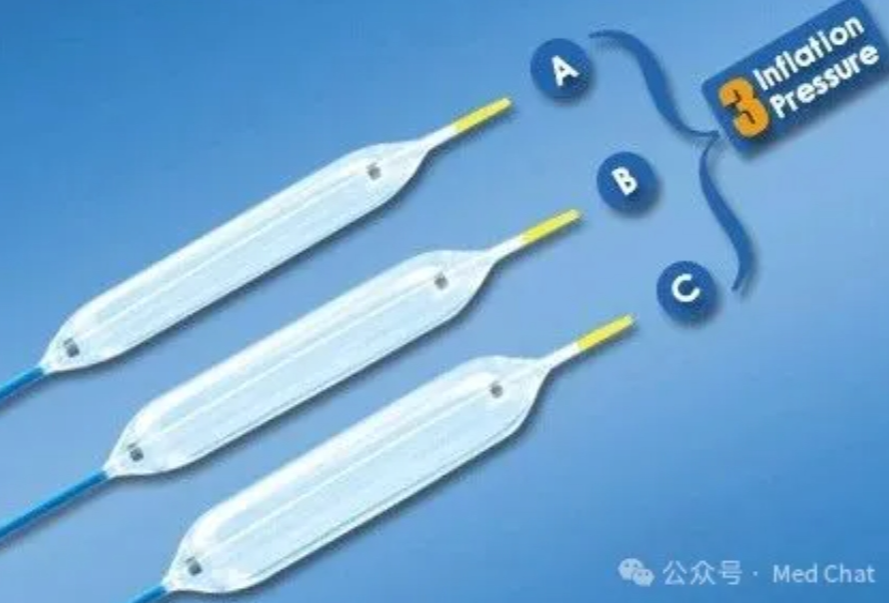
阅读更多 -
2025.08.29
中国医疗器械博览会|球管的分析报告
CT是目前医院放射科应用比较广泛的仪器之一,其全称是计算机体层成像,在多种疾病的检查与诊断中都发挥了重要作用,是十分常用的诊断手段。
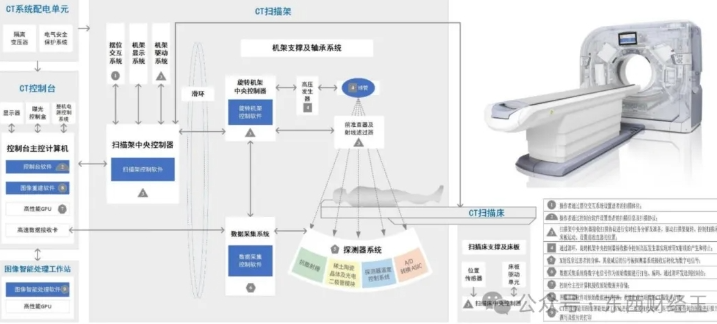
阅读更多 -
2025.08.12
2025中国医疗器械博览会|神经介入导丝,从理论到实践
本期,2025中国医疗器械博览会带您从理论到实践了解神经介入导丝。
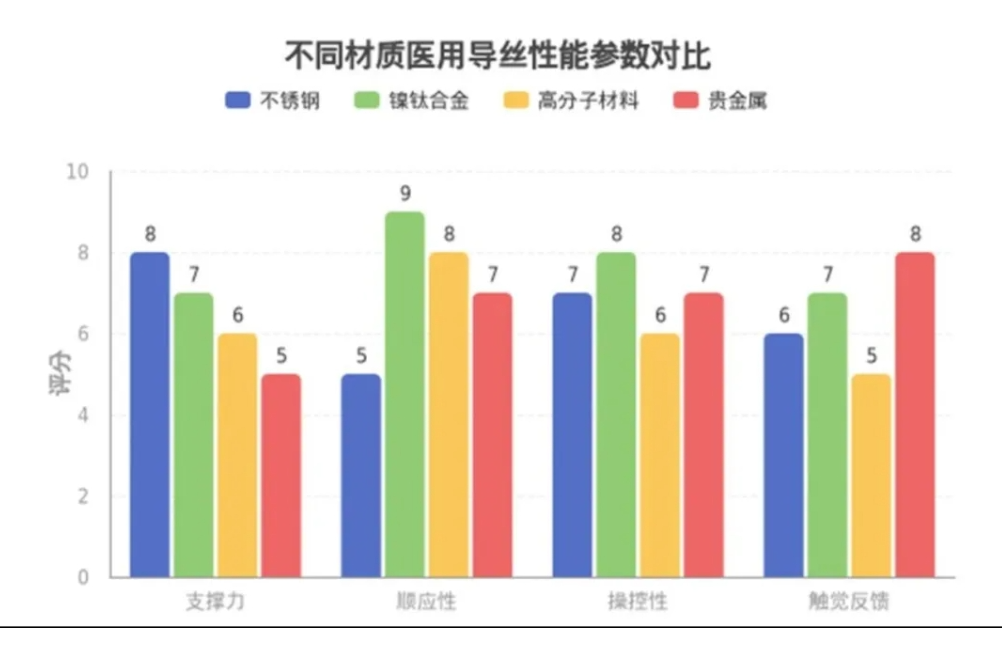
阅读更多 -
2025.07.30
上海医疗器械展|点击本文,你将了解与导丝有关的一切…
本期,上海医疗器械展带您了解与导丝有关的一切。
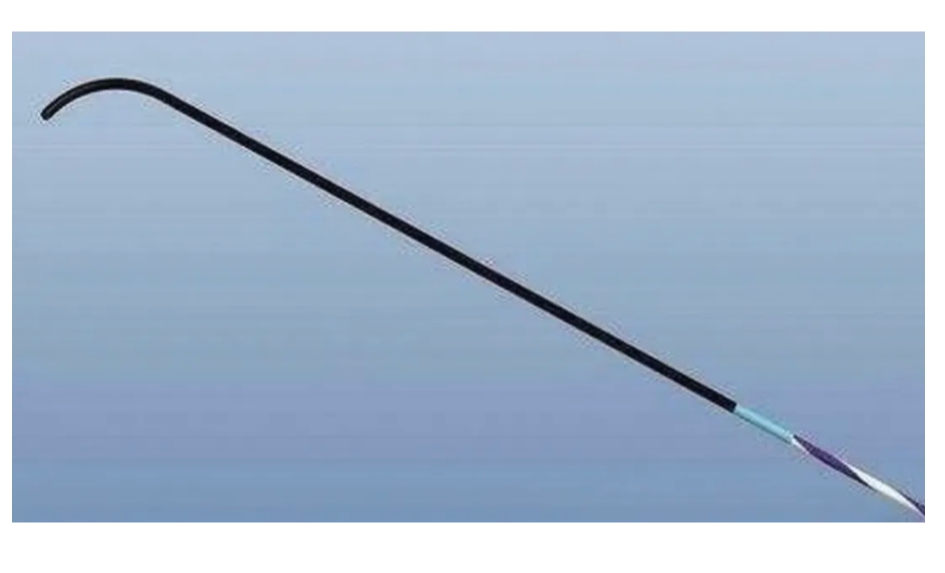
阅读更多 -
2025.07.18
中国医疗器械展|规模扩张与技术升级并行 2025 Medtec医用管件及挤压加工企业展现超强实力
全球医用管件市场正处于高速增长期,2025年市场规模预计达45.8亿美元(年复合增长率7.2%),2030年有望突破65亿美元。

阅读更多 -
2025.07.11
医疗器械博览会|文献精读—血管介入放射学中的导管:图文综述
医疗器械博览会为您介绍血管介入放射学中的导管:图文综述如下。

阅读更多 -
2025.07.09
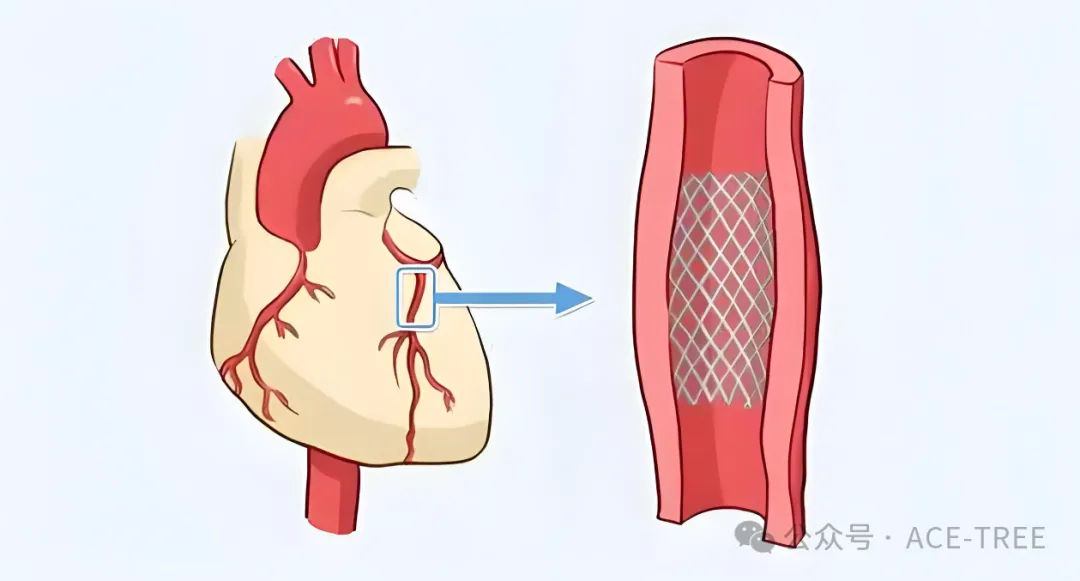
Medtec China|一文看懂心脏支架、药物球囊原理及特点
冠脉支架和药物球囊都是解决冠脉狭窄或闭塞的常用“武器”。它们就像疏通水管的两种不同工具。
阅读更多 -
2025.06.24
2025上海医疗器械展|傻傻分不清楚,医疗器械究竟有多少组1类2类的定义?
2025上海医疗器械展梳理了一下医疗器械法规标准中常见的1类和2类定义,并没有整理完全,小伙伴们可以在留言区补充。

阅读更多 -
2025.06.19
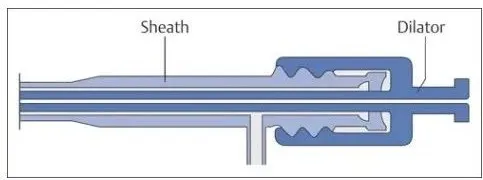
医疗器械博览会|鞘管的操作与应用安全
医疗器械博览会介绍鞘管的操作与应用安全如下:
阅读更多 -
2025.06.10
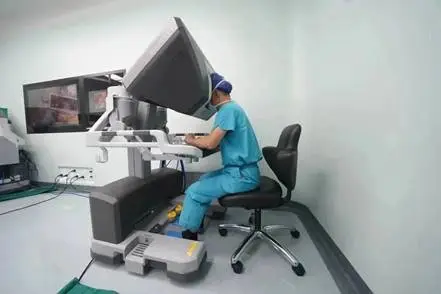
中国医疗器械博览会|手术机器人的核心组成与技术基础
近年来,手术机器人已成为医疗领域的革命性技术,从心脏搭桥到肿瘤切除,从骨科手术到神经介入,其应用场景不断拓展。
阅读更多











