质量管理
-
2025.07.02
上海医疗器械展|医疗器械注册检测常见问题及解答
以下是上海医疗器械展对医疗器械注册检测常见问题的解答。

阅读更多 -
2025.06.25
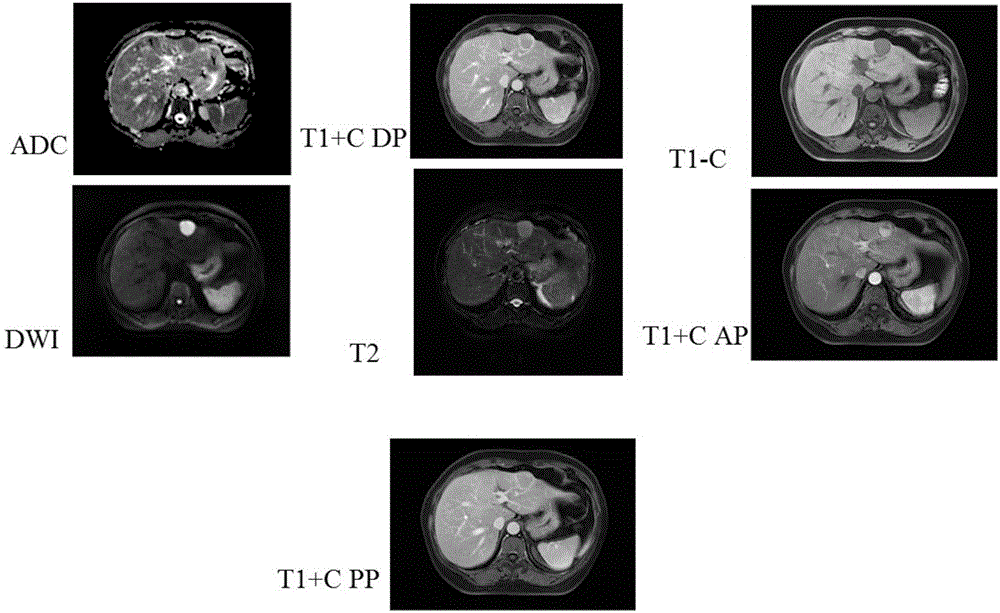
2025医疗器械展览会|MR图像质量控制的相关方法
本期2025医疗器械展览会介绍磁共振成像相关的图像质量控制方法。
阅读更多 -
2025.06.18
上海医疗器械展览会|FDA注册 | 无菌医疗器械510(k)提交资料必须包括哪些信息?
FDA发布的《标注为无菌的器械在上市前通知(510(k))提交中无菌信息的提交与审查指南》,阐明了在无菌类器械510(k) 申报资料中应包括的灭菌过程、热原信息。
阅读更多 -
2025.05.16
医疗器械博览会|EO灭菌产品追加全流程解析:标准要求与实施步骤
接上一篇EO灭菌验证三周期:短周期、半周期、全周期的标准要求与目的,医疗器械博览会在本篇将分享下灭菌产品的追加。

阅读更多 -
2025上海医疗器械展|破解运输风险!医疗器械包装测试标准选择与合规操作权威指南
医疗器械在运输过程中可能会遭遇温度、湿度或振动等各种环境因素,这些因素均可能对医疗器械的性能产生影响。

阅读更多 -
2025.04.01
2025上海医疗器械展览会|医疗器械生产质量管理之生产管理
2025上海医疗器械展览会了解到医疗器械生产质量管理之生产管理详情如下。

阅读更多 -
2025.03.26
2025中国医疗器械博览会|医疗器械质量体系文件系统检查的重点包括哪些方面?
2025中国医疗器械博览会了解到,检查员在核查时依据来自于法规同时也来自于企业制定的文件,文件系统的建立和管理是企业质量管理体系正常运行的标准,如果管理不善,必然会自食恶果。
阅读更多 -
上海医疗器械博览会|医用电气设备如何根据GB9706.1-2020来怎么确认关键元器件?
上海医疗器械博览会了解到,GB 9706.1-2020将关键元器件定义为直接影响设备基本安全和基本性能的部件,其失效可能导致设备安全风险或功能失效。

阅读更多 -
2025.03.21
上海医疗器械展览会|一文了解EO灭菌过程确认(IQ/OQ/PQ)
上海医疗器械展览会了解到,环氧乙烷(EO)灭菌过程确认是医疗器械和药品生产中的关键步骤,需确保灭菌效果可靠且符合国际标准(如ISO 11135、ISO 10993-7)。

阅读更多 -
2025.03.20
2025医疗器械展会|创新医疗器械必须走临床试验评价吗?破除误区,一文讲透合规路径
2025医疗器械展会了解到,在医疗器械行业,创新产品的研发和上市往往伴随着高昂的时间成本和资金投入,而临床试验作为关键环节,常被视为“必经之路”。
阅读更多











