金属3D打印能否助推国产牙科种植体实现进口替代
2021-03-18
在种植牙产业链中,基台和牙冠的附加值较低,而种植体是种植系统的核心部件。在我国的种植牙制造企业中,从事基台和牙冠加工的企业较多,而种植体进口产品在国内市场占有率超过90%。[1]
相比之下,国产种植体产品尚处于发展初期,精密车削加工在 CAD/ CAM 应用实例中表现和费效比并不完善,加上设计专利的短板,国产厂商在牙种植体设计制造方面难以突出重围。国产企业在品牌和渠道建设等方面与进口品牌仍有差距,与一些韩国品牌相比性价比优势并不突出[1]。在价格战策略难以奏效的情况下,通过技术创新,在产品设计、产品性能上培养竞争力,或将为国内牙科种植体企业加速实现进口替代开辟出新的道路。
根据3D科学谷的市场研究,基于选区激光熔化工艺的3D打印技术,是实现种植体设计创新的新兴途径。德国激光加工领域的专家通快(TRUMPF), 结合其选区激光熔化3D打印技术的应用,探讨了该技术在牙科种植体加工领域的应用前景,以及为国内种植体加工企业带来的机遇。
 《3D打印与牙科行业白皮书》3D科学谷
《3D打印与牙科行业白皮书》3D科学谷
技术竞争新赛道
“小螺丝钉” 中的技术挑战
牙种植体虽然看上去与普通小螺钉无异,但实际上技术含量却非常高,涉及到材料技术、数字模拟,机械设计,生物学、生物力学,临床医学等多个学科。
牙种植体与普通螺丝区别最大的一点是它的使用环境, 即它是一根钉入人体牙槽嵴的特种螺钉。
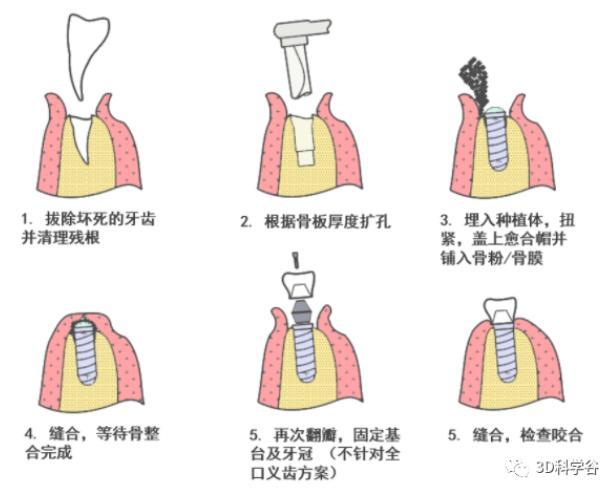 二段分离潜入式牙种植过程示意图(单颗牙种植) 通快
二段分离潜入式牙种植过程示意图(单颗牙种植) 通快
人类骨组织的维持对它承载的应力有着苛刻的要求。具体来说,种植体对周围骨组织必须施加适当的剪切力/ 压应力,辅之以骨粉/骨膜以诱发成骨过程来修复种植手术所造成的骨量损失。
种植体周围骨组织所受剪切力/压应力始终保持在植骨界面应力屏蔽的阈值之上(一般认为是 2MPa),否则骨组织没有足够承载压力就会被吸收萎缩。但同时扭力又不能过大防止引起骨损伤(一般认为是 60MPa),造成种植体周围发炎,种植体周围骨组织的持续流失。最终这根牙种植体也就摇摇欲坠,患者不得不再一次承受重新手术的痛苦。
增材制造种植体的优势
为了在种植体剪切和张拉动作对周围骨组织所施加的应力影响的狭小窗口中寻找最佳平衡,设计的诀窍是如何在粗糙孔隙表面的基础上通过表面设计获取最大植骨接触(Bone Implant Contact,BIC),最终达到最高种植体稳定系数(Implant Stability Quotient, ISQi)。
在过去的几代种植体设计中,不断改变种植体柱体壁锥形度、螺纹密度、螺距、螺深、螺形、切削口宽度,植体颈部纹路的尝试都无法彻底解决应力屏蔽和骨吸收的问题。如果想设计更复杂的造型,那么当前的车削工艺就显露出较差的费效比。而这时候增材制造就充分显示出其在实现复杂仿生结构方面的优势了。
 以色列 A.B. Dental 公司 3Di 系列种植体A.B. dental
以色列 A.B. Dental 公司 3Di 系列种植体A.B. dental
以色列牙科制造商 A.B. Dental 与 University of Delaware 大学的 Zvi Schwartz 教授联合发布了激光选区熔化 3D 打印 3Di 个性化种植体系列(Ti6Al4V-ELI基材)。
其粗糙孔隙表面模拟了自然骨的形态增加了 BIC,配合其亲水性表面增了强骨整合效果。该产品正在进行广泛的体外及临床测试,预计很快将获得注册并上市销售。
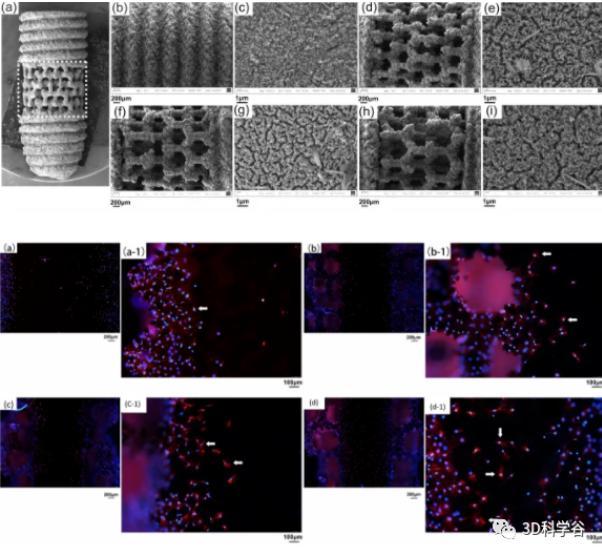 3D打印多孔支架牙种植 SEM 结果,MC3T3-E1 细胞在种植体多孔支架表面粘附荧光显微镜结果。
3D打印多孔支架牙种植 SEM 结果,MC3T3-E1 细胞在种植体多孔支架表面粘附荧光显微镜结果。
Sci Rep [2]
国内增材制造种植体研究进展
国内的科研单位在选区激光熔化 3D 打印种植体方面也颇有建树。
在 2017 年,复旦大学中山医院口腔科余优成教授研究发表了选区激光熔化 3D 打印支架结构牙种植体相关论文,旨在通过在钛合金种植体柱体上打印 100-500 微米左右的多孔支架来降低局部弹性模量,从而降低应力屏蔽的风险并优化植体轴向的应力分散。
事实表明,多孔支架能够通过改变应力传递对细胞信号道施加影响,提速成骨细胞的粘附和增殖效率,最后达到提高骨整合效率的效果。
更多来自吉林大学、上海交通大学等科研单位的的研究表明,选区激光熔化 3D 打印在未来的应用是大势所趋。
在解决种植体表面处理、内径螺丝加工的问题后,各种形态的种植体将会不断涌现。同时激光增材制造钛锆合金和其他生物陶瓷正在成为下一个技术挑战。把握住这个技术突破口,国产种植体在临床表现上的提升指日可待,这为实现牙科种植体的进口替代带来可能性。
关注医疗器械加工
德国通快集团一直重视在医疗器械行业的发展,也在医疗行业根植了多年。目前通快与高校及附属医院进行应用合作项目,通过为临床医生定制种植体 CAD 设计、选区激光熔化 3D 打印原型制造,并且提供部分后期机加工、表面处理、材料形貌表征和机械测试的渠道服务来帮助临床医生有效简化研发流程,把更多注意力放在提高医疗器械的临床表现上。同时针对医疗器械生产商,通快强化为客户提供 IQ/OQ/MQ 的能力。通过在产学研平台不断挖掘和满足客户需求来提高国产医疗器械的生产设计水平。
l参考资料
[1] 前瞻经济学人. 《2020年中国口腔医疗市场现状及发展前景分析 国产化时代来临》。
[2]Yang F, Chen C, Zhou Q, Gong Y, Li R, Li C, Klmpfl F, Freund S, Wu X, Sun Y, Li X, Schmidt M, Ma D, Yu Y. Laser beam melting 3D printing of Ti6Al4V based porous structured dental implants: fabrication, biocompatibility analysis and photoelastic study. Sci Rep. 2017 Mar 28;7:45360.
文章及图片来源:3DScienceValley











